Abstract
Background: Follicular lymphoma (FL) is the second most frequently diagnosed Non-Hodgkin lymphoma subtype in Western countries. Patients often undergo multiple lines of therapy over many years throughout the course of their disease with worse survival after each successive line of therapy. Recent findings from the ELARA trial showed that tisagenlecleucel, a chimeric antigen receptor (CAR)-T cell therapy, had durable complete response rate of 66.0%, with a probability of 79% (95% CI, 66%-87%) to remain in response ≥6 months (overall response rate 86.2%) in patients with relapsed or refractory (r/r) FL. Prior evidence in patients with r/r diffuse large B-cell lymphoma demonstrated that tisagenlecleucel can be safely infused in an outpatient setting and may reduce healthcare resource utilization (HCRU) (Lyman et al, 2020). we present the first HCRU among patients with r/r FL who received tisagenlecleucel in the ELARA trial.
Methods: ELARA is a Phase II, single-arm, multicenter study of tisagenlecleucel in adult patients with r/r FL. All patients underwent lymphodepleting chemotherapy with fludarabine and cyclophosphamide or bendamustine, before receiving a single IV infusion of tisagenlecleucel (0.6-6×10 8 CAR-positive viable T cells) that was administered at the investigator's discretion in either the inpatient or outpatient setting. Patients were followed for a median of 11 months, and HCRU was characterized using hospitalization data collected from the first day of infusion up to the second month after treatment, the time period wherein occurrence of CAR-T cell-related adverse events (AEs) such as cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome are most frequent. Information on the length of stay (dates of admission), hospital facilities used, and discharge information were assessed. Healthcare costs associated with hospitalizations and intensive care unit (ICU) admissions were estimated by applying unit costs obtained from published literature. All costs were from healthcare system perspective and were inflated to 2020 US Dollars.
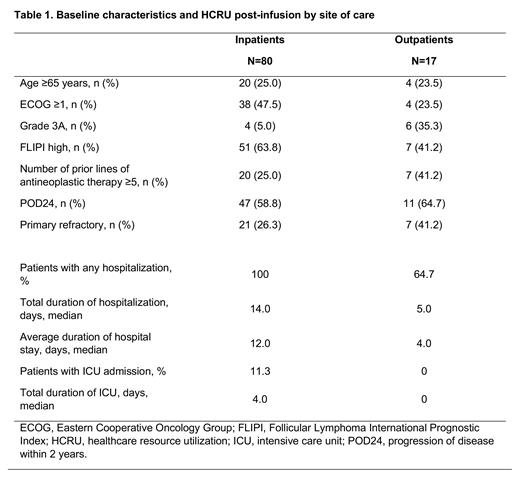
Results: Among 97 patients with r/r FL who received tisagenlecleucel infusion, 17 patients (18%) were infused in an outpatient setting and 80 patients (82%) were admitted for inpatient infusion and monitoring. Of the 30 clinical trial sites, 4 sites in US and 1 site in Australia used outpatient administration; at these sites, 68% (17 of 25) of the patients were treated in an outpatient setting. Patients treated in the outpatient setting were more likely to have ECOG performance status of 0 and a more favorable FLIPI score, but more frequently had grade 3A FL, primary refractory disease, and >5 lines of prior antineoplastic therapy than in the inpatient setting (Table). In the outpatient setting, 6 of 17 patients (35%) did not require any hospitalization during the first 2 months post-infusion, whereas 11 of 17 patients (65%) were hospitalized for AEs at a median of 3 days (range 1-25) post-infusion. Patients treated in the inpatient setting had longer total hospitalization days (mean ± SD: 14.3 ± 8.42 vs 5.0 ± 2.16 days) and longer average length of stay (mean ± SD: 13.8 ± 8.54 vs 4.3 ± 1.4 days) compared with the outpatient group. None of the outpatients required ICU admission during the 2 months post-infusion, whereas 7 patients (9%) in the inpatient setting were admitted to the ICU, for a total mean ± SD duration of 5.6 ± 4.47 days (Table). Mean overall hospitalization costs for inpatients were $40,054 per patient, which included $36,351 for inpatient stays and $3,703 for ICU, and $7,477 per patient for outpatients, which are only for inpatient stays as there were no ICU stays.
Conclusions: In the ELARA trial, hospitalization and ICU patterns varied substantially between inpatient and outpatient settings and favored HCRU in the outpatient setting. Among patients treated in the outpatient setting, one third of patients did not require hospitalization during the post-infusion period; those who did had a lower average length of stay than the patients infused in an inpatient setting. The mean hospitalization costs in the post-infusion period were substantially lower in the outpatient versus inpatient setting due to the lack of ICU admissions. These data suggest that tisagenlecleucel can be safely administered in the outpatient setting, which may reduce HCRU for patients with r/r FL.
Clinical trial information: NCT03568461
Fowler: BostonGene, Corp: Current Employment, Current holder of stock options in a privately-held company; Bristol Myers Squibb, F. Hoffmann-La Roche Ltd, TG Therapeutics and Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Dickinson: Gilead Sciences: Consultancy, Honoraria, Speakers Bureau; MSD: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Takeda: Research Funding; Celgene: Research Funding; Amgen: Honoraria; Roche: Consultancy, Honoraria, Other: travel, accommodation, expenses, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Chen: Mesolbast: Honoraria; Morphosys: Honoraria. Andreadis: GenMAB: Research Funding; Epizyme: Honoraria; Atara: Consultancy, Honoraria; Crispr Therapeutics: Research Funding; Novartis: Research Funding; BMS/Celgene: Research Funding; Karyopharm: Honoraria; Incyte: Honoraria; Kite: Honoraria; Merck: Research Funding; Roche: Current equity holder in publicly-traded company, Ended employment in the past 24 months; TG Therapeutics: Honoraria. Tiwari: Novartis Healthcare private limited: Current Employment. Masood: Novartis: Current Employment, Current holder of stock options in a privately-held company. Ramos: Novartis: Current Employment, Current equity holder in publicly-traded company. Bollu: Novartis: Current Employment, Current equity holder in publicly-traded company. Jousseaume: Novartis: Current Employment, Current equity holder in publicly-traded company. Thieblemont: Kyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses , Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Hospira: Research Funding; Bayer: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses . Dreyling: BeiGene: Consultancy; Astra Zeneca: Consultancy, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau; Abbvie: Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; Amgen: Speakers Bureau; Genmab: Consultancy; Incyte: Consultancy, Speakers Bureau; Gilead/Kite: Consultancy, Research Funding, Speakers Bureau; Bayer HealthCare Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau. Schuster: Celgene: Consultancy, Honoraria, Research Funding; Nordic Nanovector: Consultancy; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Abbvie: Consultancy, Research Funding; Acerta Pharma/AstraZeneca: Consultancy; Alimera Sciences: Consultancy; BeiGene: Consultancy; Juno Theraputics: Consultancy, Research Funding; Loxo Oncology: Consultancy; Tessa Theraputics: Consultancy; Genentech/Roche: Consultancy, Research Funding; Pharmaclyclics: Research Funding; Adaptive Biotechnologies: Research Funding; Merck: Research Funding; Incyte: Research Funding; TG Theraputics: Research Funding; DTRM: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal